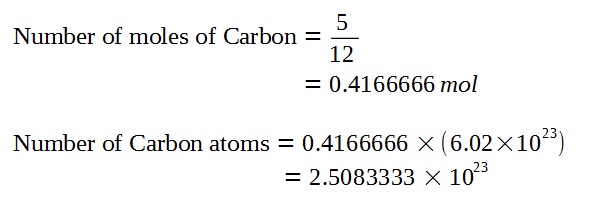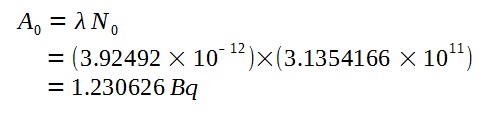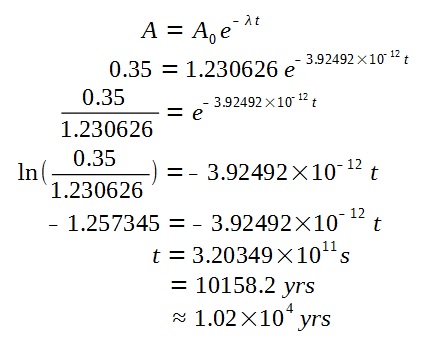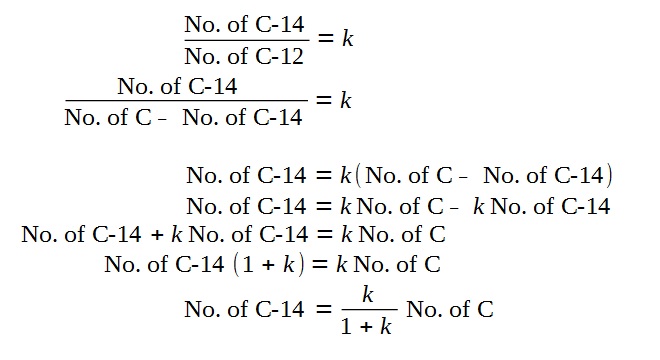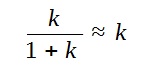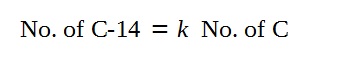Question
A piece of furniture was recovered from an archaeological site and carbon dating was used to estimate its age. It was assumed that the proportion of Carbon-14 to natural carbon (Carbon-12) in living wood was 1.25 x 10-12. If the number of particles emitted from 5 g of carbon prepared from the specimen was 21 per minute, how old is the specimen?
[The half-life of Carbon-14 is 5600 years and the mass number of natural carbon is 12.]
Answer
Since the proportion of Carbon-14 is very small compared to Carbon-12, we can assume for the sake of finding the number of carbon atoms that all of the carbon in the specimen is Carbon-12.
Given that the proportion of Carbon-14 to Carbon-12, we can find the original number of Carbon-14 atoms when the piece of furniture was living wood using the following approximation. (Refer to bottom for further discussion of this approximation.)
Using the half-life, we can calculate the decay constant of Carbon-14.
Hence, we can find the initial activity when the specimen was still living wood.
The final activity of the specimen is given by
Now, we can find the age of the specimen.
Discussion on Approximation
Based on the phrasing of the question that
“the proportion of Carbon-14 to natural carbon is k”,
However, since the k is small compared to 1,
Hence, we arrive at